For example consider the regions of the 1h nmr spectrum of vinyl acetate shown below.
Nmr of vinyl acetate.
Vinyl acetate is used to make other industrial chemicals.
Vinyl acetate has been used as an acyl donor for enzyme catalyzed acylation reactions.
For that nmr was chosen as a.
Vinyl acetate 108 05 4 nmr spectrum vinyl acetate h nmr spectral analysis vinyl acetate c nmr spectral analysis ect.
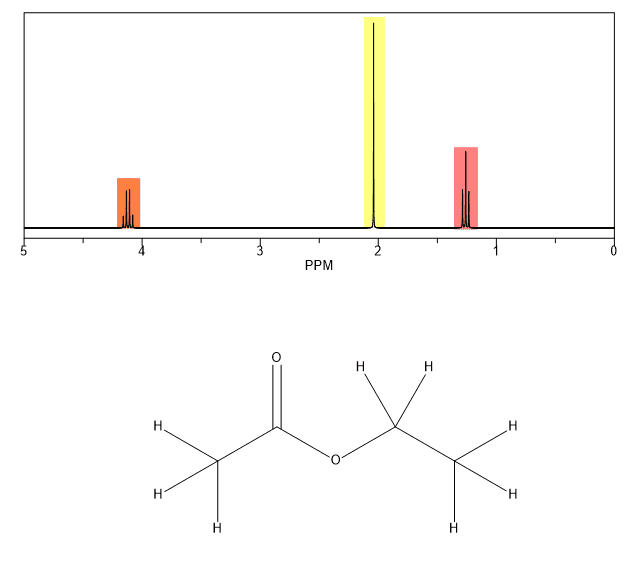
Ethyl acetate exists as a solid soluble in water and an extremely weak basic.
Spectroscopy that permits to obtain information on both chemical structure.
Materials with a distinct vinyl acetate ratio.
Vinyl acetate is an industrial chemical that is produced in large amounts in the united states.
Chemicalbook providevinyl acetate 108 05 4 1h nmr ir2 ms ir3 ir1 1h nmr raman esr 13c nmr spectrum.
The three inequivalent protons of the vinyl group labeled ha hb and hc do not appear as the type of multiplets we saw above.
Packaging 1 2 5 l in glass bottle 18 l in steel drum 25 ml in glass bottle safety documentation.
It is a clear colorless liquid with a sweet fruity smell.
Acetate is a monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid it has a role as a human metabolite and a saccharomyces cerevisiae metabolite.
7 nmr and 8 ftir.
Rather they are each a doublet of doublets which is a direct result of the j values of each proton.
Ethyl acetate also known as 1 acetoxyethane or acetic ester belongs to the class of organic compounds known as carboxylic acid esters.
It is very flammable and may be ignited by heat sparks or flames.